Rattan,
Fibre from the stems of various climbing palms of the genus Calamus, used for matting and basketry.
Fibre from the stems of various climbing palms of the genus Calamus, used for matting and basketry.
Ravanhattha,
Stringed fiddle-like instrument played by a bhopa in Rajasthan whilst narrating the Pabuji epic.
Stringed fiddle-like instrument played by a bhopa in Rajasthan whilst narrating the Pabuji epic.
Raw Silk/Wild Silk,
The wild or raw silk is obtained from silkworms known as the tassar, muga and eri as well as some other local varieties is indigenous to Indis It is well known for its textural beauty and subdued tones. Tassar worms are simply fed on any tree in the hilly tracts of the country and produce a variety of silk that is stiff in texture. The muga worm is reared mainly in Assam where it is fed on a species of laurel, producing a golden-hued silk. Eri worms feed on the leaves of the castor plant, locally known as arundee, and produce a silk which is rough-textured and lacks a glossy shine.
The wild or raw silk is obtained from silkworms known as the tassar, muga and eri as well as some other local varieties is indigenous to Indis It is well known for its textural beauty and subdued tones. Tassar worms are simply fed on any tree in the hilly tracts of the country and produce a variety of silk that is stiff in texture. The muga worm is reared mainly in Assam where it is fed on a species of laurel, producing a golden-hued silk. Eri worms feed on the leaves of the castor plant, locally known as arundee, and produce a silk which is rough-textured and lacks a glossy shine.
Ream,
Ream is a unit of measurement that means 500 sheets. For examples, Can I have a ream of A4 paper.
Ream is a unit of measurement that means 500 sheets. For examples, Can I have a ream of A4 paper.
Red Woods,
Camwood, Barwood, Sanderwood (Santal, Sandal, Red Sanders), Brazil wood, Sapan wood, Peach wood.
Camwood, Barwood, Sanderwood (Santal, Sandal, Red Sanders), Brazil wood, Sapan wood, Peach wood.
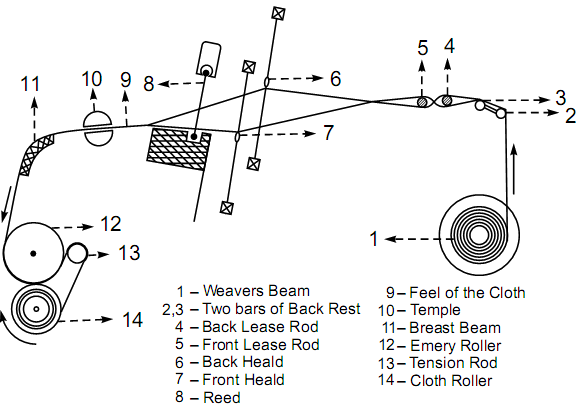
Reed,
Reed is a comb like frame that pushes the weft yarn on the hand loom firmly against the finished cloth after each insertion. A piece of loom apparatus consisting usually of fine /wood slivers standing vertically between two horizontal bars. The comb acts as a warp-spacer and, when weaving, the weaver beats the comb against the newly inserted weft thread with her sword.
Reed is a comb like frame that pushes the weft yarn on the hand loom firmly against the finished cloth after each insertion. A piece of loom apparatus consisting usually of fine /wood slivers standing vertically between two horizontal bars. The comb acts as a warp-spacer and, when weaving, the weaver beats the comb against the newly inserted weft thread with her sword.
Refinery,
The refinery was a separate hearth used to convert grey cast iron into white cast iron prior to its conversion to wrought iron in the finery and chafery. With the introduction of the coke fired blast furnace the silicon content of cast iron increased. This resulted in the production of grey cast iron rather than white, as was normally the case with the charcoal-fired blast furnace. Grey cast iron was more difficult to refine, because the silicon had to be oxidized before the carbon could be removed. The carbon in grey cast iron was in the form of graphite was more difficult to oxidize than when it was combined with iron in the form of cementite, as in white cast iron. To solve this problem, an additional step in the fining process was added, in which the grey cast iron was heated under oxidizing conditions until the silicon was removed and the metal was converted to white cast iron.
The refinery was a separate hearth used to convert grey cast iron into white cast iron prior to its conversion to wrought iron in the finery and chafery. With the introduction of the coke fired blast furnace the silicon content of cast iron increased. This resulted in the production of grey cast iron rather than white, as was normally the case with the charcoal-fired blast furnace. Grey cast iron was more difficult to refine, because the silicon had to be oxidized before the carbon could be removed. The carbon in grey cast iron was in the form of graphite was more difficult to oxidize than when it was combined with iron in the form of cementite, as in white cast iron. To solve this problem, an additional step in the fining process was added, in which the grey cast iron was heated under oxidizing conditions until the silicon was removed and the metal was converted to white cast iron.
Refining,
Refining is the separation of a metal from its impurities. As such it is applied to a wide range of different processes for the different metals. Copper The copper that was produced by the last stage of the smelting process was, in general, rather impure (black copper with copper content as low as 90%). This had a high iron content, which would have to be reduced by two stage refining process if the copper was to be useable for most purposes. This was done by first an oxidizing process to convert the metallic iron in the metal to iron oxide (usually magnetite), and then a reducing process (poling) to remove the excessive amount of oxygen introduced by the iron removal stage. Refining black copper The magnetite would float on top of the metal where it could either be slagged by the addition of silica and tapped off, or the pasty magnetite could be physically scrapped off the top of the liquid metal to form a high magnetite smithing hearth bottom type slag. Unfortunately, the tapped refining slag would be difficult to distinguish from other of copper smelting slags, or even iron smelting slag - however the presence of a significant amount of partially digested crushed quartz fragments is often an indicator of a copper smelting or refining slag. The high magnetite smithing hearth bottoms, or skulls, have been recorded rarely from archaeological contexts. This is almost certainly because the number of sites on which refining occurs would be limited as - • Either the slag would be produced on the original smelting site, where the small amount of such slag produced would be swamped by the much large amount of 'normal' smelting slag. Thus not easily recovered and recorded. • Or the refining occurred away from the smelting site, that is, the copper was traded as black copper ingots. In this case, the slag might be mistaken as a slightly unusual iron smithing slag. Poling This was the final stage of the refining process designed to reduce the oxygen content of copper to a reasonable level. It was carried out by plunging a pole of wood into a bath of molten copper this produces hydrogen and other reducing gases by the distillation of the wood. These in turn reduce any copper oxide present. Alloying copper with tin or zinc would also deoxidise the metal but at the expense of loss of expensive alloying element to slag or vapour. It is, therefore, more economical to pole the metal before alloying. Silver The main method of separating silver from the majority of its impurities was by cupellation of the impure metal with lead. However, cupellation would not separate gold from silver. Gold Cupellation Cupellation was used to separate gold from those alloys containing copper but as silver is not oxidized during the cupellation process, it would not separate gold from silver. Thus, if high purity gold was required for jewellery, or coinage the metal would have to be further refined by parting. Mercury amalgam refining In the classical period mercury amalgamation refining was used to recover and refine gold from worn out gold embroideries and gold leaf gilding. Gold dissolves in the mercury to form a pasty amalgam. The majority of the mercury was remove by squeezing the mixture chamois leather leaving the amalgam behind. The gold was then recovered by heating amalgam so that the remaining mercury was lost by evaporation. The same technique was later used to recover finely divided gold from crushed ore. Parting To get rid of silver from gold it was necessary to use a parting technique. The earliest of the heated the impure gold with salt in combination with other chemicals to convert the silver to silver chloride which diffused out of the metal. This method appears to be first used to produce the gold for the Lydian coinage produced at Sardis in the 6th century BC (Rampage and Craddock 2000). Later other parting methods were used to treat impure gold alloys; these included acid, and the antimony or sulphide parting. Related term: Parting Iron Bloomery iron and steel did not undergo a specific refining process other than by forging and possibly folding and welding to break up the slag inclusions and homogenize the metal. However, as the major use of iron was in the malleable form, the majority of cast iron was (re)fined to wrought iron by oxidizing carbon, and when present the silicon, out of the metal. Initially in Britain, this was carried out in the finery forge using the Walloon process where both a finery and chafery hearth were used. After Henry Cort’s use of the reverberatory furnace to convert the iron and grooved rolls to convert the resultant bloom to bar, the puddling process generally replaced the finery forge.
Refining is the separation of a metal from its impurities. As such it is applied to a wide range of different processes for the different metals. Copper The copper that was produced by the last stage of the smelting process was, in general, rather impure (black copper with copper content as low as 90%). This had a high iron content, which would have to be reduced by two stage refining process if the copper was to be useable for most purposes. This was done by first an oxidizing process to convert the metallic iron in the metal to iron oxide (usually magnetite), and then a reducing process (poling) to remove the excessive amount of oxygen introduced by the iron removal stage. Refining black copper The magnetite would float on top of the metal where it could either be slagged by the addition of silica and tapped off, or the pasty magnetite could be physically scrapped off the top of the liquid metal to form a high magnetite smithing hearth bottom type slag. Unfortunately, the tapped refining slag would be difficult to distinguish from other of copper smelting slags, or even iron smelting slag - however the presence of a significant amount of partially digested crushed quartz fragments is often an indicator of a copper smelting or refining slag. The high magnetite smithing hearth bottoms, or skulls, have been recorded rarely from archaeological contexts. This is almost certainly because the number of sites on which refining occurs would be limited as - • Either the slag would be produced on the original smelting site, where the small amount of such slag produced would be swamped by the much large amount of 'normal' smelting slag. Thus not easily recovered and recorded. • Or the refining occurred away from the smelting site, that is, the copper was traded as black copper ingots. In this case, the slag might be mistaken as a slightly unusual iron smithing slag. Poling This was the final stage of the refining process designed to reduce the oxygen content of copper to a reasonable level. It was carried out by plunging a pole of wood into a bath of molten copper this produces hydrogen and other reducing gases by the distillation of the wood. These in turn reduce any copper oxide present. Alloying copper with tin or zinc would also deoxidise the metal but at the expense of loss of expensive alloying element to slag or vapour. It is, therefore, more economical to pole the metal before alloying. Silver The main method of separating silver from the majority of its impurities was by cupellation of the impure metal with lead. However, cupellation would not separate gold from silver. Gold Cupellation Cupellation was used to separate gold from those alloys containing copper but as silver is not oxidized during the cupellation process, it would not separate gold from silver. Thus, if high purity gold was required for jewellery, or coinage the metal would have to be further refined by parting. Mercury amalgam refining In the classical period mercury amalgamation refining was used to recover and refine gold from worn out gold embroideries and gold leaf gilding. Gold dissolves in the mercury to form a pasty amalgam. The majority of the mercury was remove by squeezing the mixture chamois leather leaving the amalgam behind. The gold was then recovered by heating amalgam so that the remaining mercury was lost by evaporation. The same technique was later used to recover finely divided gold from crushed ore. Parting To get rid of silver from gold it was necessary to use a parting technique. The earliest of the heated the impure gold with salt in combination with other chemicals to convert the silver to silver chloride which diffused out of the metal. This method appears to be first used to produce the gold for the Lydian coinage produced at Sardis in the 6th century BC (Rampage and Craddock 2000). Later other parting methods were used to treat impure gold alloys; these included acid, and the antimony or sulphide parting. Related term: Parting Iron Bloomery iron and steel did not undergo a specific refining process other than by forging and possibly folding and welding to break up the slag inclusions and homogenize the metal. However, as the major use of iron was in the malleable form, the majority of cast iron was (re)fined to wrought iron by oxidizing carbon, and when present the silicon, out of the metal. Initially in Britain, this was carried out in the finery forge using the Walloon process where both a finery and chafery hearth were used. After Henry Cort’s use of the reverberatory furnace to convert the iron and grooved rolls to convert the resultant bloom to bar, the puddling process generally replaced the finery forge.
Refractory,
The term either describes the heat resisting properties of a material, or the heat resistant objects such as tuyères, crucibles, and furnace and hearth linings. A refractory material must have the following properties. A high melting point It is likely that much of the history of metallurgy has been controlled by the improvement of the properties of refractories. Although most copper alloy metallurgy can be carried out in the temperature range in which common clay-base refractories are adequate, the same is not true for the production of steel and cast iron. Chemically inert with respect to the charge At high temperatures the slag produced can attack some furnace linings extremely aggressively. This was particularly true in the 19th and 20th centuries with the development of the basic steel making. The normal silica refractories were attacked and destroyed rapidly by the basic (calcium-rich) slag needed to reduce the phosphorus content of the steel. Mechanical strength and dimensional stability at high temperatures. In the case of crucibles, the material must be able to support the weight of the molten metal and be able to be picked up without excess deformation at the pouring temperature. In the case of furnace linings they must be able to support the weight of the material above them without slumping. Thermal shock resistance Crucibles, in particular, have to with stand large thermal shocks when the crucible is removed from the furnace for pouring. Furnace linings have to withstand thermal stresses imposed by thermal gradients within the furnace lining. Thermal conductivity Ideally crucibles and muffles should have high conductivity, whereas, furnace linings should have low conductivity. However, conductivity of crucible refractory was not factor that would have been considered important until recently, the other factors being much more important. Similarly, for furnace linings the requirement for low thermal conductivity was not an overriding factor, although reducing the overall thermal losses through the furnace walls would have been important. The difference between the thermal conductivity of modern refractories and those used in the past has not always been considered in some experimental reconstructions of early smelting processes. The use of different refractory materials can have a major effect on the thermal losses and thus on the blowing and rate of fuel use required to run a smelt. The earliest refractories were made from local clays. These may have been modified to improve their properties usually by the addition of quartz rich sand, but where available graphite was used. Eventually, some clays (high kaolin fire-clays) were discovered to make particularly good refractories. So that production became centralized in a few centres and the crucible exported over large distances as is shown by the import of large number of Hessian crucibles, manufactured in what is now Germany, into England during the 17th and 18th centuries. The introduction of the blast furnace required the use of refractories with high silica content to withstand the higher temperatures involved. These were either in the form of natural high purity sandstones with low iron content, or later silica bricks that contain more than 96% silica. To deal with basic slag or higher temperatures refractories based on magnesite MgO or dolomite (Ca,Mg)CO3 were used.
The term either describes the heat resisting properties of a material, or the heat resistant objects such as tuyères, crucibles, and furnace and hearth linings. A refractory material must have the following properties. A high melting point It is likely that much of the history of metallurgy has been controlled by the improvement of the properties of refractories. Although most copper alloy metallurgy can be carried out in the temperature range in which common clay-base refractories are adequate, the same is not true for the production of steel and cast iron. Chemically inert with respect to the charge At high temperatures the slag produced can attack some furnace linings extremely aggressively. This was particularly true in the 19th and 20th centuries with the development of the basic steel making. The normal silica refractories were attacked and destroyed rapidly by the basic (calcium-rich) slag needed to reduce the phosphorus content of the steel. Mechanical strength and dimensional stability at high temperatures. In the case of crucibles, the material must be able to support the weight of the molten metal and be able to be picked up without excess deformation at the pouring temperature. In the case of furnace linings they must be able to support the weight of the material above them without slumping. Thermal shock resistance Crucibles, in particular, have to with stand large thermal shocks when the crucible is removed from the furnace for pouring. Furnace linings have to withstand thermal stresses imposed by thermal gradients within the furnace lining. Thermal conductivity Ideally crucibles and muffles should have high conductivity, whereas, furnace linings should have low conductivity. However, conductivity of crucible refractory was not factor that would have been considered important until recently, the other factors being much more important. Similarly, for furnace linings the requirement for low thermal conductivity was not an overriding factor, although reducing the overall thermal losses through the furnace walls would have been important. The difference between the thermal conductivity of modern refractories and those used in the past has not always been considered in some experimental reconstructions of early smelting processes. The use of different refractory materials can have a major effect on the thermal losses and thus on the blowing and rate of fuel use required to run a smelt. The earliest refractories were made from local clays. These may have been modified to improve their properties usually by the addition of quartz rich sand, but where available graphite was used. Eventually, some clays (high kaolin fire-clays) were discovered to make particularly good refractories. So that production became centralized in a few centres and the crucible exported over large distances as is shown by the import of large number of Hessian crucibles, manufactured in what is now Germany, into England during the 17th and 18th centuries. The introduction of the blast furnace required the use of refractories with high silica content to withstand the higher temperatures involved. These were either in the form of natural high purity sandstones with low iron content, or later silica bricks that contain more than 96% silica. To deal with basic slag or higher temperatures refractories based on magnesite MgO or dolomite (Ca,Mg)CO3 were used.
Registers of Traditional Knowledge,
Registers can be analyzed from many different perspectives. According to their legal nature, registers can be termed either declarative or constitutive, depending upon the system under which they are established. A declaratory regime relating to traditional knowledge recognizes that the rights over traditional knowledge do not arise due to any act of government but rather are based upon pre–existing rights, including ancestral, customary, moral and human rights. In the case of declarative registers, although registration does not affect the existence of such rights, it may be used to assist patent officials in analyzing prior art, and to support challenges to patents granted which may have directly or indirectly made use of traditional knowledge. In circumstances where these registers are organized in an electronic form and available through the Internet, it is important to establish a mechanism that ensures that entry dates of traditional knowledge are valid when carrying out searches related to novelty and inventiveness. A third function that these registers may have is to facilitate benefit–sharing between users and providers. Constitutive registers form part of a legal regime which seeks to grant rights over traditional knowledge. Constitutive registers will record the granting of rights (i.e. exclusive property rights) to the traditional knowledge holder as a means to ensure their moral, economic and legal interests are protected and recognized. Most model constitutive registers are conceived as public in nature, run by a national entity and under a law or regulation which clearly determines how valid registration of traditional knowledge can take place and be formally recognized and accepted. As such they may be more controversial and difficult to design and face some critical challenges and questions in moving from concept to practice. As an example of a national law, Article 16 of the Peruvian Law N° 27811 “Law Introducing a Protection Regime for the Collective Knowledge of Indigenous Peoples Derived from Biological Resources” provides that “[t]he purposes of the Registers of Collective Knowledge of Indigenous Peoples shall be the following, as the case may be: (a) to preserve and safeguard the collective knowledge of indigenous peoples and their rights therein; (b) to provide INDECOPI with such information as enables it to defend the interests of indigenous peoples where their collective knowledge is concerned.” Article 15 also provides that “[t]he collective knowledge of indigenous peoples may be entered in three types of register: (a) Public National Register of Collective Knowledge of Indigenous Peoples; (b) Confidential National Register of Collective Knowledge of Indigenous Peoples; (c) Local Registers of Collective Knowledge of Indigenous Peoples.”
Registers can be analyzed from many different perspectives. According to their legal nature, registers can be termed either declarative or constitutive, depending upon the system under which they are established. A declaratory regime relating to traditional knowledge recognizes that the rights over traditional knowledge do not arise due to any act of government but rather are based upon pre–existing rights, including ancestral, customary, moral and human rights. In the case of declarative registers, although registration does not affect the existence of such rights, it may be used to assist patent officials in analyzing prior art, and to support challenges to patents granted which may have directly or indirectly made use of traditional knowledge. In circumstances where these registers are organized in an electronic form and available through the Internet, it is important to establish a mechanism that ensures that entry dates of traditional knowledge are valid when carrying out searches related to novelty and inventiveness. A third function that these registers may have is to facilitate benefit–sharing between users and providers. Constitutive registers form part of a legal regime which seeks to grant rights over traditional knowledge. Constitutive registers will record the granting of rights (i.e. exclusive property rights) to the traditional knowledge holder as a means to ensure their moral, economic and legal interests are protected and recognized. Most model constitutive registers are conceived as public in nature, run by a national entity and under a law or regulation which clearly determines how valid registration of traditional knowledge can take place and be formally recognized and accepted. As such they may be more controversial and difficult to design and face some critical challenges and questions in moving from concept to practice. As an example of a national law, Article 16 of the Peruvian Law N° 27811 “Law Introducing a Protection Regime for the Collective Knowledge of Indigenous Peoples Derived from Biological Resources” provides that “[t]he purposes of the Registers of Collective Knowledge of Indigenous Peoples shall be the following, as the case may be: (a) to preserve and safeguard the collective knowledge of indigenous peoples and their rights therein; (b) to provide INDECOPI with such information as enables it to defend the interests of indigenous peoples where their collective knowledge is concerned.” Article 15 also provides that “[t]he collective knowledge of indigenous peoples may be entered in three types of register: (a) Public National Register of Collective Knowledge of Indigenous Peoples; (b) Confidential National Register of Collective Knowledge of Indigenous Peoples; (c) Local Registers of Collective Knowledge of Indigenous Peoples.”
Relief,
Type of sculpture in which figures are raised above the surface or from background that is flat or has hollowed out parts.
Type of sculpture in which figures are raised above the surface or from background that is flat or has hollowed out parts.
Relief Polishing,
During sample preparation for metallography, there is a tendency for the softer phases in a sample to be preferentially removed by the abrasive paste. Normally this effect is not wanted, but may be helpful in revealing phosphorus ghost structures in phosphoritic irons.
During sample preparation for metallography, there is a tendency for the softer phases in a sample to be preferentially removed by the abrasive paste. Normally this effect is not wanted, but may be helpful in revealing phosphorus ghost structures in phosphoritic irons.